The National Agency for Food and Drug Administration and Control (NAFDAC) has issued a public warning concerning a falsely labeled anti-diabetic drug, Ozempic, circulating in markets.
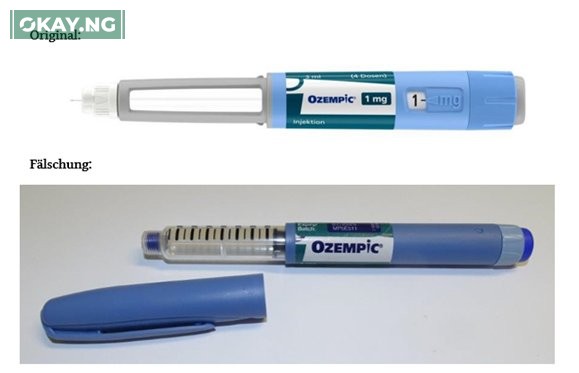
The agency raised alarm about the presence of Ozempic, a solution for injection drug, contained in pre-filled pens, which have been identified in international markets.
In a post via its official X handle, NAFDAC disclosed that Ozempic is not registered with the agency. However, the drug has become scarce in the markets globally due to its high demand, leading to falsification.
The post reads, “NAFDAC is informing the public about the circulation of pre-filled pens falsely labeled as the diabetes medicine, Ozempic (semaglutide, 1 mg, solution for injection), which was identified at wholesalers in the European Union and the UK as reported by the European Medicines Agency.”
It further stated, “The reports of falsification came in the wake of an increase in demand for Ozempic, which has led to a global shortage situation. The pens have batch numbers, 2D barcodes, and unique serial numbers from genuine Ozempic packs.”
While emphasizing that Ozempic is not registered by NAFDAC, the agency expressed concern that the product might have been distributed in Nigeria through informal markets. NAFDAC cautioned Nigerians to purchase all medical products from authorized/licensed suppliers and to confirm the authenticity of such products.
Ozempic is used to treat type-2 diabetes and is also employed in long-term weight management as an anti-obesity medication. Additionally, it is utilized to lower the risk of heart attack, stroke, or death in patients with type-2 diabetes and heart or blood vessel disease.
The tweet concluded, “NAFDAC implores importers, distributors, retailers, and healthcare providers and patients to always exercise caution and vigilance within the supply chain to avoid the importation, distribution, sale, and administration or use of falsified or substandard medicinal products.”